






Frequently Recommended Calcaneus Ultrasound Bone Densitometer for Bone Health Assessment
SKU: UMY-US-034
Brand: Umy
MOQ: 1
Origin: China
Quick Info.
- SKU NO.: UMY-US-034
- Device Classification: Class Ⅱ
- Warranty: 1 Year
- Power Source: Electric
- Transport Package: Carton
- Origin: China
- Material: Metal, Plastic
- After-Sale Service: Online Technical Support
- Production Capacity: 6000 Sets/Year
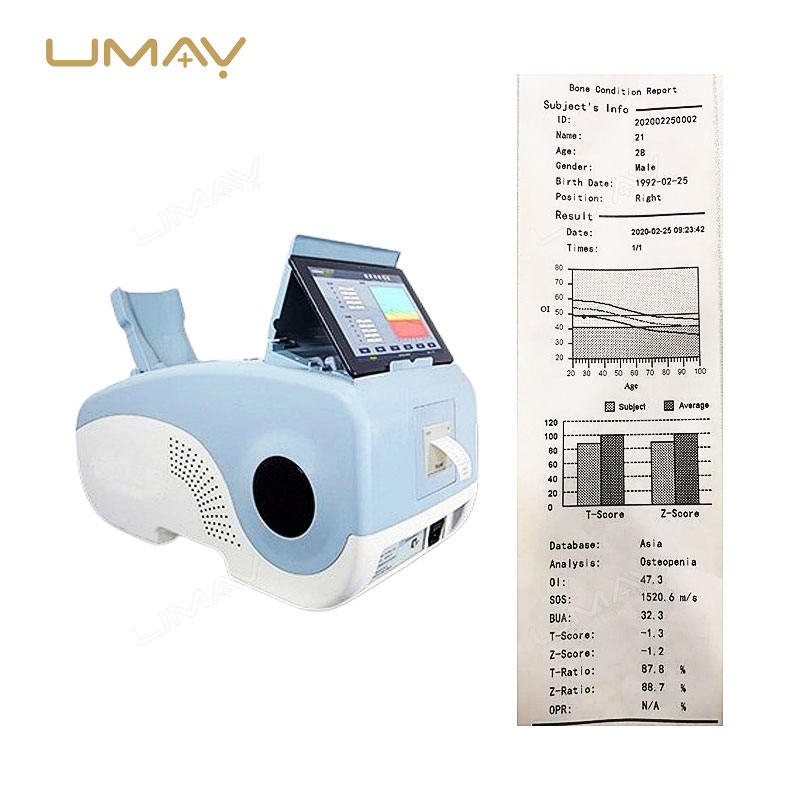
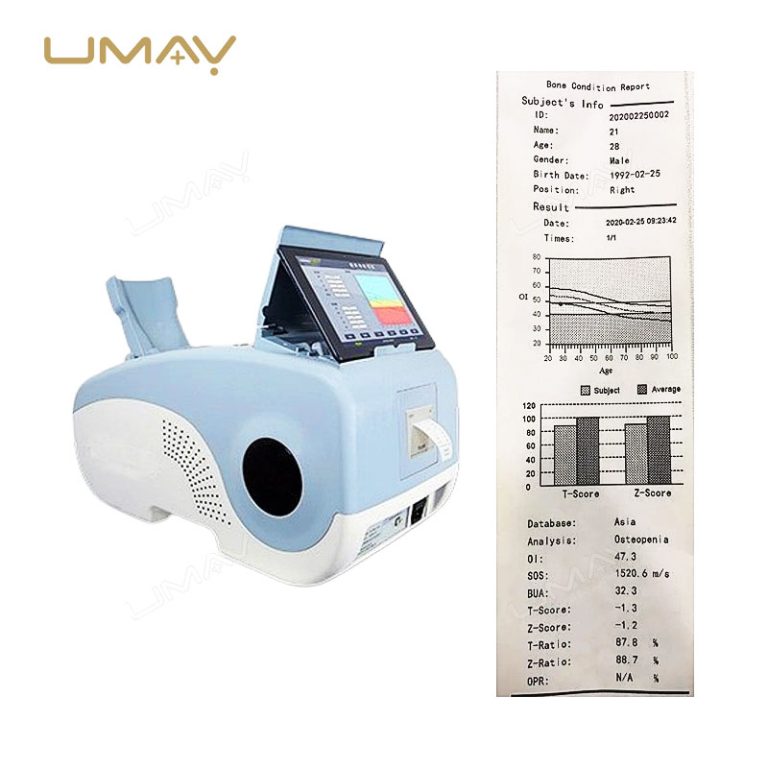
The calcaneus ultrasound bone densitometer is a non-invasive tool designed to assess bone density in the heel, providing vital information for bone health evaluation. Using advanced ultrasound technology, it helps detect early signs of osteoporosis and monitor bone strength over time. This portable, high-quality device offers fast and accurate results, making it ideal for routine screenings and diagnostic purposes. Its compact design ensures ease of use in both clinical and hospital settings. Trusted by healthcare professionals, it plays a crucial role in preventing bone fractures and managing conditions like osteoporosis.
The Specific Parameters
| ITEMS | PARAMETERS |
|---|---|
| Ultrasonic Parameters | |
| BUA | Ultrasonic amplitude attenuation |
| SOS | Ultrasonic sound velocity |
| OI | Bone mass index |
| Measurement Method | |
| Type | Full dry type (no liquid inside and outside the equipment, no temperature control required) |
| Transmission | Two-way ultrasonic transmission and reception |
| Probe Specifications | |
| Frequency | 0.5MHz ± 10% |
| Broadband (at 4-6dB) | >60% |
| Measurement Details | |
| Measurement Time | ≤ 25 seconds |
| Test Repeatability (OPR) | ≤ ±1% |
| Measurement Accuracy (SOS) | ≤ ±2% |
| Test Repeatability (BUA) | ≤ ±5% |
| Diagnostic Parameters | |
| Parameters | BUA, OI value, T value, Z value, SOS, OPR, adult ratio, age ratio, obesity index, height prediction, BMI, child Z value chart |
| Ultrasonic Output | |
| TIS | 2.8 × 10^-3 mW/cm² |
| Calibration | |
| Correction | Automatic calibration of the human body simulation module |
| Temperature Compensation | |
| System | Automatically compensates for measurement deviation caused by temperature |
| Reference Database | |
| Regions | Asian, European, Middle Eastern, and African databases |
| Interface and Output | |
| Interface | Dual USB, external tablet, built-in thermal printer, external printer support |
| Report Types | Diagnostic report, PACS network system-compatible |
| Measurement Site | |
| Probe Spacing | Automatically adjusts for direct contact with the heel |
| Bone Density Software | |
| System | Child and adult bone density test software, automatic probe location and best signal search |
| Prompts | Automatic test site placement prompts |
| Parameters | |
| Adults | T value, Z value, age ratio, adult ratio, OPR, OI, SOS, BUA |
| Children | Z value, BMI, height prediction, obesity, SOS, BUA |
| Additional Features | |
| Animation Playback | Attracts children’s attention during examination |
| Probe | Permanent oil bladder probe, no replacement required |
| Interface and Reports | |
| Language Switching | Chinese/English interface |
| Report Formats | A4, B5, and others, preview and print options |
| Bluetooth | Supports Bluetooth report transmission |
| Supports WeChat scan code self-download | |
| Networking | |
| Internet Functions | Supports hospital network systems and expert remote consultation |
| Data Management | |
| Case Database | Automatic recording, query, classification, backup; export results to Excel |
| Environmental Requirements | |
| Operating Humidity | 30-70% (non-condensing) |
| Operating Temperature | 10-40°C |
| Power Requirements | |
| Voltage | AC220V ± 10% |
| Frequency | 50Hz |
| Power | 3.15A, 125W |
| Instrument Specifications | |
| Weight | 13kg |
| Size (W×H×L) | 330mm × 360mm × 645mm |